Abstract
Introduction: In older and/or unfit patients with newly diagnosed AML, the median duration of response to azacitidine+venetoclax (Aza/Ven) is 17.5 months. However, there is wide variability in duration of response, with some patients relapsing quickly and others with highly durable responses. Although several factors including measurable residual disease negativity and the presence of an NPM1 and/or IDH2 mutation have been associated with durable responses, it is currently difficult to predict which patients may achieve very prolonged remissions with Aza/Ven. This uncertainty may be stressful for patients and can make decisions regarding other available therapies such as allogeneic stem cell transplant challenging. We hypothesized that specifically studying the very long-term responders to Aza/Ven (an outlier group we have termed "Super Responders") may identify predictors of durable responses.
Methods: To investigate predictors of very long-term response to Aza/Ven, charts of patients with newly diagnosed AML treated with Aza/Ven between January 2015 and December 2021 at a single institution were reviewed. Baseline patient demographic and clinical data was recorded, and responses to Aza/Ven were assessed according to ELN criteria. Patients who proceeded to transplant after responding to Aza/Ven were excluded from this analysis. To identify a group of Super Responders, event-free survival (EFS) was calculated and outliers were identified by Interquartile Range (IQR) box plot analysis. Clinical and disease characteristics of the Super Responders were then compared to the Non-Super Responders using chi-square or Fisher's exact tests for categorical variables and t-tests or Wilcoxon tests for continuous variables. Approval for this retrospective study was obtained from the Institutional Review Board.
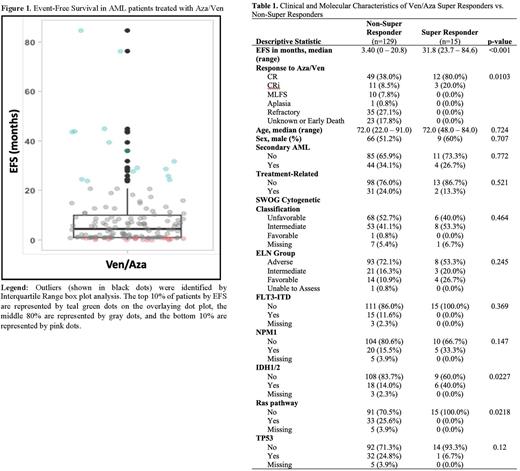
Results: Charts from 184 patients treated with frontline Aza/Ven for newly diagnosed AML were reviewed. After excluding 40 patients who proceeded to allogeneic transplant after response to Aza/Ven, 144 patients were analyzed. Fifteen outliers (termed Super Responders) with the longest EFS were identified by box plot analysis (Figure 1). These 15 patients also represented the top 10% of EFS (represented by green dots in Figure 1). The n=15 Super Responders had a median EFS of 31.8 months (range 23.7 to 84.6 months) compared to a median EFS of 3.4 months (range 0 to 20.8 months) among the n=129 Non-Super Responders (p<0.001). There was no significant difference in the frequency of secondary or treatment-related AML, SWOG cytogenetic classification, or ELN risk group when comparing the Super Responders to the Non-Super Responders (Table 1). Regarding molecular profile, mutations identified in Super Responders included: TET2 (n=7), SRSF2 (n=5), NPM1 (n=5), IDH2 (n=4), RUNX1 (n=2), IDH1 (n=2), DNMT3A (n=2), PHF6 (n=2), ASXL1 (n=2), TP53 (n=1), SF3B1 (n=1), and FLT3-TKD (n=1). The Super Responders had a higher incidence of IDH mutations, 40% vs 14% (p = .02). The rate of NPM1 mutations was not significantly different between groups, although 0% of subjects with NPM1 mutations in the Super Responder group had concomitant FLT3-ITD mutations compared to 40% in the Non-Super Responder group. No Ras pathway mutations were identified in Super Responders.
Conclusions: Traditional prognostic factors including ELN risk group, cytogenetic classification, and having secondary or treatment-related AML do not predict whether a patient will have a highly durable response to Aza/Ven. Consistent with prior reports, IDH mutations (especially IDH2 mutations) are more common in Super Responders. NPM1 mutations did not appear to be more common in Super Responders in our analysis but this may be related to not controlling for the presence of a co-occurring FLT3-ITD mutation. Notably, no Super Responders had FLT3-ITD or Ras pathway mutations, consistent with prior data suggesting that Ras mutations may contribute to resistance to Aza/Ven. Multivariate analyses and analyses looking at the role of co-occurring mutations and measurable residual disease in predicting the likelihood of highly durable response to Aza/Ven are ongoing.
Disclosures
Smith:AML JV: Research Funding; Kura: Research Funding; Syros: Research Funding; Argenx: Research Funding. Pollyea:Syndax: Consultancy; Bristol-Myers Squibb: Consultancy; Genentech: Consultancy; Gilead Sciences: Consultancy; Takeda Oncology: Consultancy; Syros: Consultancy; Celgene: Consultancy; AbbVie: Consultancy, Research Funding; Aprea: Consultancy; Foghorn: Consultancy; Kiadis: Consultancy; Novartis: Consultancy; Astellas: Consultancy; Teva: Research Funding. McMahon:Syros: Research Funding; Arcellx: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal